We may earn revenue from the products available on this page and participate in affiliate programs. Learn More ›
Q: During a recent blackout, my neighbor and I kicked around the idea of alternative power sources. Besides solar and wind, he mentioned fuel cells, which I hadn’t heard much about. What is a fuel cell, how is it used, and how is it different from other energy sources?
A: A fuel cell creates electricity through a process that transforms energy created from a chemical reaction between a fuel (usually hydrogen) and an oxidizing agent (typically oxygen). Though fuel cells aren’t used as widely as more traditional power generation sources, such as fossil fuels or lithium-ion batteries, they have a broad range of applications. Although they do not need recharging like batteries, they do need a fuel supply to produce electricity.
In transportation, fuel cells power cars, trucks, trains, and even submarines. You’ll also find them serving as primary energy sources for residential and commercial buildings in rural areas that are off the grid. Hospitals, data centers, and even grocery stores use them as backup power generation solutions, ensuring the businesses have electricity in the event of a power failure.
What is a fuel cell?
Unlike a lithium-ion battery, which stores energy, a fuel cell creates chemical energy through an electrochemical reaction between fuel (usually hydrogen) and the oxygen in the surrounding air. When hydrogen and oxygen atoms combine, the reaction releases a massive amount of energy. In fact, the chemical reaction between the two elements is so powerful that it can send rockets into space.
When this energy is routed through a fuel cell (instead of being used to create an explosion), the reaction creates electricity and heat. When the chemical reaction is large enough, it can produce enough electricity to move a vehicle or provide power to a building.
How does a fuel cell work?
Fuel cells use four basic parts to create energy: the anode, cathode, electrolyte, and catalyst. In a hydrogen fuel cell, the most common type, hydrogen atoms enter the fuel cell at the anode. Once inside the anode, a catalyst, usually platinum, separates the hydrogen molecules into electrons and protons.
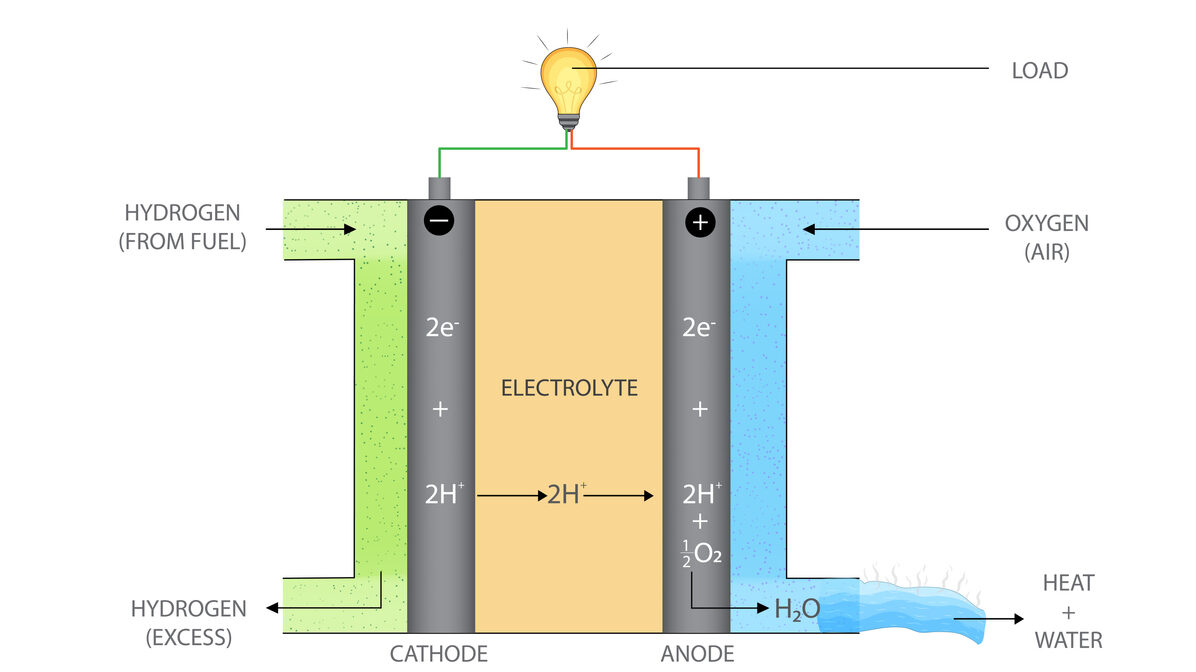
The electrons are then forced through a circuit, creating electricity that can power an electric car, hospital, or apartment building. After exiting the circuit, the electrons arrive at the cathode, where they reunite with the protons and combine with oxygen to create the reaction’s byproducts of water and heat.
What are the benefits of fuel cells?
Fuel cells have several eye-popping benefits. They burn no fossil fuels, produce no pollution, and get twice the mileage of battery-powered cars. (We should note that the process of creating the necessary hydrogen for fuel cells isn’t 100-percent clean but is comparable to lithium-ion battery emissions and far cleaner than burning fossil fuels). Whereas lithium-ion batteries take hours to recharge, you can refill a tank of hydrogen in 5 minutes or less, similar to the time needed to fill a gas tank with unleaded fuel.
Given these benefits, why are we seeing so many battery-powered Teslas on the road and very few hydrogen cars? Many states haven’t invested in hydrogen refueling stations because they’re so expensive to build. It costs nearly $2 million to build a hydrogen refueling station. As such, there are currently fewer than 70 hydrogen refueling stations in the country, nearly all of which are in California. In comparison, you can charge a car powered by a lithium-ion battery by plugging it into a standard power outlet at home.
Fuel cells see more widespread use as stationary power sources. They’re far more efficient than traditional power plants that burn fossil fuels. According to the U.S. Department of Energy, fuel cell systems can generate electricity with efficiencies of up to 60 percent, far greater than the 33 to 35 percent efficiency of a traditional power plant.
RELATED: Solved! Are Solar Panels Really Worth The Cost?

7 Types of Fuel Cells to Know
While the polymer electrolyte membrane (PEM) may be the most common among the types of fuel cells, there are six other examples of fuel cells that have paved the way for today’s fuel cell technology, are currently in use, or are under development:
- Polymer Electrolyte Membrane (PEM): The most common type of fuel cell is smaller and lighter than other fuel cells. PEM fuel cells, which run off of hydrogen, water, and oxygen, are lightweight compared to other fuel cells and operate at a relatively low maximum temperature of about 176 degrees Fahrenheit. With these characteristics, hydrogen fuel cells are the ideal choice for fuel cell electric vehicles.
- Direct Methanol: Instead of using hydrogen as its fuel source, this type of fuel cell uses methanol mixed with water. Methanol has a higher energy density than hydrogen and is a liquid, which makes it easier to transport while giving the user more energy from a smaller amount of fuel. As such, direct methanol fuel cells are ideal for portable electronics, such as cell phones and laptop computers.
- Alkaline (AFCs): one of the first fuel cell technologies, AFCs have been used to provide much of the power for the onboard systems on NASA spacecraft for more than half a century. However, since exposure to carbon dioxide can negatively impact the performance of an AFC, this type of fuel cell isn’t as widely used on planet Earth.
- Solid Oxide: Solid oxide fuel cells use a ceramic compound to create electricity. While they’re 60 percent efficient at converting energy to electricity, which is comparatively higher than other fuel cell types, they operate at temperatures that can eclipse 1,800 degrees Fahrenheit. As such, they require significant heat shielding to protect the people and materials around them. While this makes solid oxide suitable as backup generators to data centers and other sensitive buildings, solid oxide is not an option for vehicles or smaller electronics.
- Phosphoric Acid: This type of fuel cell uses phosphoric acid as its catalyst with carbon electrodes that contain a platinum catalyst. Phosphoric isn’t particularly efficient at creating electricity, with an efficiency rating of between 37 percent and 42 percent. As a result, makers of these phosphoric acid fuel cells compensate by building them larger than other fuel cell types. Their bulky size limits this type of fuel cell for use mainly as a stationary power source.
- Molten Carbonate: This type of fuel cell uses an electrolyte that consists of a molten carbonate salt mixture with metal as a catalyst. They have a high 65 percent efficiency rating and don’t require a precious metal as a catalyst, and therefore are cost-effective fuel cells. With these characteristics, molten carbonate fuel cells are good choices for utilities. However, since they operate at high temperatures, their components degrade more quickly, giving them a shorter lifespan than other fuel cells.
- Reversible: This type of fuel cell functions like a PEM fuel cell by converting the reaction between oxygen and hydrogen into energy. When combined with solar and wind power, reversible fuel cells can use the excess energy created on sunny or windy days to split water, the byproduct of a PEM fuel cell, into oxygen and hydrogen, hence creating its own fuel. While still in development, reversible fuel cells present a truly renewable energy resource.



